P-Stim Fraud: A New DOJ Enforcement Priority?

The Department of Justice regularly publicizes its fraud prevention and False Claims Act enforcement priorities. These announced priorities typically focus on broad issues that affect the lives of millions of Americans – COVID-19 fraud, the opioid crisis, and the rapid expansion of telehealth. In addition, we keep an eye on DOJ enforcement actions, and these can reveal emerging trends, often in narrow areas. One such area has become apparent over the past year: the fraudulent misbilling of electro-acupuncture devices also referred to as peri-auricular stimulation device or P-Stims, which are taped to patients’ ears as surgical implants.
What is a P-Stim?
Sold under brand names such as ANSiStim, E-pulse, Stivax, and NeuroSti, P-Stim devices are used for acupuncture, usually to fight a patient’s chronic pain. A P-stim, as shown here,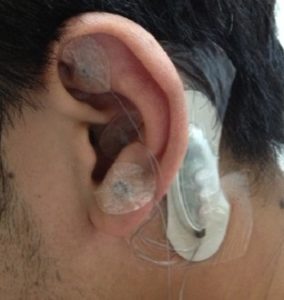 consists of a small electronic stimulator and three wires, with small acupuncture needles on the end. The stimulator is placed behind a patient’s ear and the needles pierce the skin on the front side of the ear. The device is taped into place and generally remains with a patient for a period of five days. After five days, the device is thrown away and the patient may get a new one.
consists of a small electronic stimulator and three wires, with small acupuncture needles on the end. The stimulator is placed behind a patient’s ear and the needles pierce the skin on the front side of the ear. The device is taped into place and generally remains with a patient for a period of five days. After five days, the device is thrown away and the patient may get a new one.
P-Stim Billing Fraud
As said, the P-Stim device is used in acupuncture. However, Medicare and other government healthcare programs do not pay for acupuncture or for acupuncture devices such as P-Stim. So, how are providers billing government healthcare programs for the P-Stim?
Healthcare providers generally bill the Medicaid and Medicare programs by submitting a reimbursement code, known as a common procedure terminology, or CPT code. The CPT code at the center of this fraud scheme is L8679, for which the government pays between $5,000 and $6,500 per occurrence.
L8679 is defined as an “implantable neurostimulator, pulse generator, any type.” This billing code is properly used for implanted medical devices that stimulate a patient’s spine. Like the P-Stim, these devices are also used to treat chronic pain. Unlike the P-Stim, the devices under L8679 are not taped to a patient’s ear; instead, they are surgically implanted into (or near) a patient’s spine, under anesthesia.
The image here, 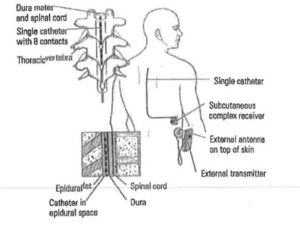 taken from a complaint the State of Tennessee filed against a psychiatrist under that state’s Medicaid False Claims Act, depicts a device that would be properly billed under L8679. It is clear to any layperson that this is a very different medical device than the P-Stim.
taken from a complaint the State of Tennessee filed against a psychiatrist under that state’s Medicaid False Claims Act, depicts a device that would be properly billed under L8679. It is clear to any layperson that this is a very different medical device than the P-Stim.
Unlike the P-Stim, devices billed under L8679 are covered by Medicare, Medicaid, and TRICARE. Because the P-Stim cannot properly be billed using L8679 and is not otherwise approved for reimbursement by government healthcare programs, any Medicare, Medicaid, or TRICARE reimbursement for these devices is improper.
Recent P-Stim Enforcement Actions
There have already been several settlements centering on P-Stim billing fraud in 2021, with the most recent one announced Monday.
- April 19, 2021: A family doctor in Maryland agreed to pay over $660,000 to resolve allegations that for five months of 2019, his practice improperly billed for P-Stim procedures.
- January 12, 2021: A spinal clinic in Texas agreed to pay over $330,000 to resolve allegations that 41 P-Stim devices were improperly billed to Medicare.
- January 4, 2021: A chiropractor practice in Tennessee agreed to pay over $1.7 million to resolve allegations that the practice improperly billed Medicare and Medicaid for P-Stim devices for a period of over two years.
These 2021 recoveries build on prior busy years, with the government previously settling at least five P-Stim cases in places including Texas, Georgia, and Pennsylvania with provider types including chiropractors, pain management physicians, and anesthesiologists.
Key Takeaways Regarding P-Stim Billing Fraud
Given the geographical scope of the recent settlements and the variety of provider types that have been involved in these cases, combatting fraudulent P-Stim billing is clearly an area of enforcement focus for the Department of Justice.
Interestingly, none of the recent settlements have come out of cases filed by whistleblowers. This is in stark contrast with the general trend of the government’s recoveries from healthcare fraud schemes, where whistleblowers are responsible for over 70% of the money the government recovers under the False Claims Act.
The current settlements are likely just the tip of the iceberg, and P-Stim billing fraud is probably endemic in our system. Whistleblowers should take this government enforcement trend as a call to action: if you see P-Stim fraud, say something. You can help save taxpayers millions, and you may be eligible to receive a share of the government’s recovery.
Read More:
- Healthcare Fraud
- Medical Billing Fraud
- The False Claims Act
- Government Enforcement Actions
- Whistleblower FAQs
- Contact us for a confidential consultation
Tagged in: Chiropractic, FCA Federal, Healthcare Fraud, Medical Billing Fraud, Medical Devices and DME, Provider Fraud,