July 27, 2016
Connecticut psychiatrist Dr. Anton Fry and his company
CPC Associates agreed to pay $36,704 to resolve allegations they violated the False Claims Act by submitting improper claims to Medicare for psychiatric services that were provided over the phone instead of by meeting with the beneficiaries in the office and treating them in person. The allegations originated in a whistleblower lawsuit filed under the
qui tam provisions of the False Claims Act by Jodi Cohen, a former patient of Dr. Fry, and Medical Bill Consultants, LLC, a billing company. They will receive a whistleblower award of $6,239 from the proceeds of the government's recovery.
DOJ (DCT)
 By the C|C Whistleblower Lawyer Team
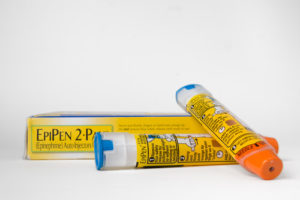
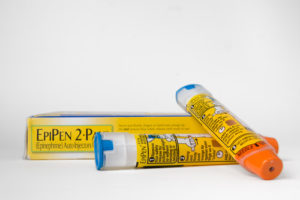
Last Friday, the Department of Justice (“DOJ”) announced a $465 million settlement with Mylan, Inc. to resolve claims that it violated the False Claim Act. The suit was brought by a whistleblower under the False Claims Act qui tam provisions and involved the alleged improper misclassification of the EpiPen as a generic drug to avoid paying rebates owed mainly to Medicaid....
By the C|C Whistleblower Lawyer Team
Last Friday, the Department of Justice (“DOJ”) announced a $465 million settlement with Mylan, Inc. to resolve claims that it violated the False Claim Act. The suit was brought by a whistleblower under the False Claims Act qui tam provisions and involved the alleged improper misclassification of the EpiPen as a generic drug to avoid paying rebates owed mainly to Medicaid....
 By the C|C Whistleblower Lawyer Team
This week's Department of Justice "Catch of the Week" goes to Mylan Inc. and Mylan Specialty L.P. Yesterday, the pharmaceutical companies agreed to pay $465 million to settle charges they violated the
By the C|C Whistleblower Lawyer Team
This week's Department of Justice "Catch of the Week" goes to Mylan Inc. and Mylan Specialty L.P. Yesterday, the pharmaceutical companies agreed to pay $465 million to settle charges they violated the  By the C|C Whistleblower Lawyer Team
A $2.5M
By the C|C Whistleblower Lawyer Team
A $2.5M  By the C|C Whistleblower Lawyer Team
In 2012, an anonymous
By the C|C Whistleblower Lawyer Team
In 2012, an anonymous  By the C|C Whistleblower Lawyer Team
This week's Department of Justice "Catch of the Week" goes to Tennessee-based defense contractor Kilgore Flares Company. On Monday, the company and one of its subcontractors, New York-based ESM Group Inc., agreed to pay $8 million to resolve charges they violated the
By the C|C Whistleblower Lawyer Team
This week's Department of Justice "Catch of the Week" goes to Tennessee-based defense contractor Kilgore Flares Company. On Monday, the company and one of its subcontractors, New York-based ESM Group Inc., agreed to pay $8 million to resolve charges they violated the